Stroke or cerebrovascular accident is a syndrome caused by disruption in the blood flow to part of the brain, most commonly due to occlusion of a blood vessel (ischemic stroke). Interruption of the blood flow deprives the brain of nutrients and oxygen, leading to neuronal cell death and often resulting in motor and sensory deficits, memory and reasoning abnormalities, coma, and even death.
Every year ~616,000 Americans and ~880,000 Europeans experience new or recurrent ischemic stroke. The global acute ischemic stroke market was estimated at $2.9 billion in 2008, and has been predicted to grow to $3.6 billion by 2015. Recombinant tissue plasminogen activator (rTPA) is the only FDA-approved for ischemic stroke drug.
However, rTPA’s narrow therapeutic window, need to exclude hemorrhagic stroke, and requirement for specific training severely limit its application to less than 5% of stroke patients. Thus, developing a safe and effective anti-stroke therapeutic agent will fulfill an unmet need and have the potential to occupy a significant portion of the market.
Preclinical studies:
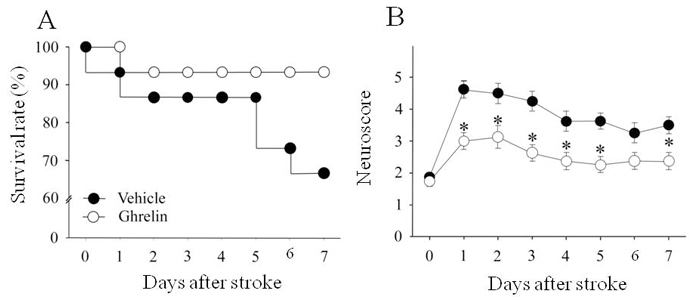
Ghrelin increases survival and function after ischemic stroke. Treatment with human ghrelin increased survival from 66 to 93% after acute ischemic stroke. Vehicle (saline) or ghrelin were administered intravenously in equal volumes starting immediately after middle cerebral artery occlusion with a loading bolus (ghrelin = 44 µg/kg) followed by continuous infusion (ghrelin = 44 µg/kg/d) over the 7-day period. Survival rates were estimated using the Kaplan-Meier method (n=15/group), and compared using the log-rank test; P = 0.07 vs. vehicle. (B) Treatment with human ghrelin resulted in sustained improvement in neurological function, as assessed by the beam balance test, over the 7-day period compared with the vehicle group. Data are expressed as mean ± SE (n=8/group) and analyzed by Kruskal-Wallis one-way ANOVA on ranks with the Student-Newman-Keuls method; * P < 0.05 vs. vehicle.
